
Clinical Research
We undertake:-
We undertake:-
|
●
|
Healthy Volunteer Studies
|
|
●
|
Clinical projects to evaluate and/or compare wound healing therapies ranging from Case Studies to multi-
|
|
●
|
Clinical projects to generate chronic wound-
|
Our services include:-
|
●
|
Sourcing & evaluation of investigators/centres
|
|
●
|
Preparation of study documentation
|
|
▪
|
Protocol
|
|
▪
|
Case Record Forms
|
|
▪
|
Patient Information Sheet
|
|
▪
|
Patient Consent Form
|
|
●
|
Ethics submission & liaison (UK National Research Ethics Service)
|
|
●
|
Negotiation with UK-
|
|
●
|
Study Monitoring & logistics
|
|
●
|
Preparation of study reports for internal use or peer review publication
|
|
●
|
Image (& acetate tracing) analysis of clinical trial wound photographs
|



Example
|
•
|
Multiple (10) case study evaluation of a new foam dressing under short-
|
|
•
|
Purpose – to support out-
|
|
•
|
Product status – CE marked device
|
|
•
|
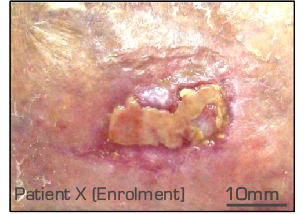
Wound type – venous ulcer
|
|
•
|
Wound status
|
|
v
|
low to moderate exudation
|
|
v
|
non-
|
|
v
|
> 3 months duration
|
|
•
|
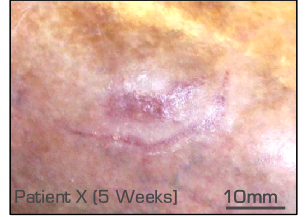
Assessments (over 6 weeks)
|
|
v
|
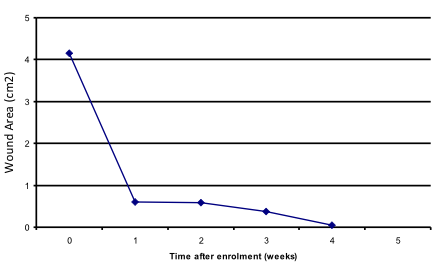
Closure (image analysis)
|
|
v
|
Pain (between & at dressing change) -
|
|
v
|
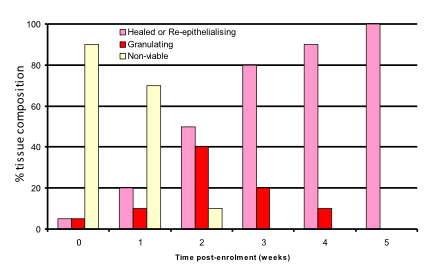
Tissue composition of wound (image analysis)
|
|
v
|
Peri-
|
|
v
|
Ease of use (relative to comparable product)
|
|
v
|
Adverse effects
|
|
•
|
Findings
|
|
v
|
Dressing performed well.
|
|
v
|
Considered equivalent to similar products – by clinician
|
|
v
|
No adverse effects
|
|
•
|
Outcome
|
|
v
|
Product has been out-
|

We can provide microbiological analysis of clinical trial samples (wound biopsies or surface wound swabs) using conventional microbiological techniques.