
|
•
|
Sub-
|
Basics
|
•
|
Test materials compared to standards & competitor materials
|
|
•
|
Surface photography of implantation sites at regular intervals
|
|
•
|
Implant site assessed at various time points after implantation
|
|
•
|
Gross inspection & documentation (e.g. photography) of implant sites
|
|
•
|
Histological/biochemical assessment to determine bio-
|
|
•
|
Documentation of adverse/unexpected events through the study
|
|
•
|
Study period – up to a maximum of 12 months
|
Assessments
Bio-Compatibility - assessment of the tissue response to the presence of the implant and/or any degradation products – in terms of:-
|
v
|
extent & duration of the inflammatory response
|
|
v
|
extent & form of cellular ingress
|
|
v
|
extent of foreign body reaction development
|
|
v
|
extent of peri-
|
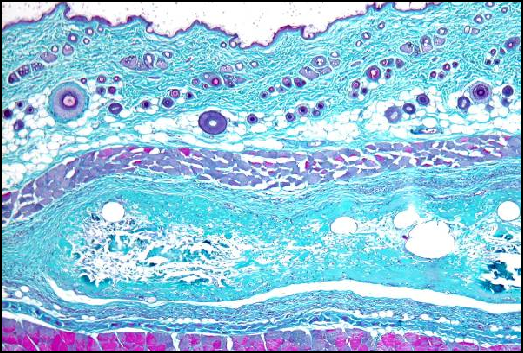
Bio-Degradability - assessed in terms of the amount of implant material remaining with time after implantation. Determined from gross and/or histological images
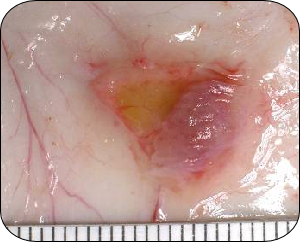
No response to product A
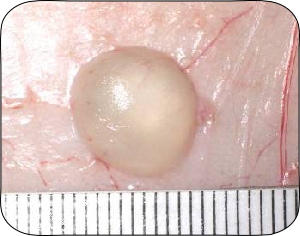
Significant response to product B
Applications
|
•
|
Widely used bio-
|
|
•
|
Valuable performance & safety data
|
|
•
|
Bio-
|
|
•
|
The impact of inadvertent entrapment of dressing materials in wounds
|
Navigation
Pre-clinical research -Bio-Compatibility & Bio-Degradation
Wound Healing Models
Physical Testing
Protease Regulation / Modulation
Anti-Microbial Efficacy Testing
Haemostat Assessment
Bio-compatibility / degradation
Back